Fluorescent Mice Herald Gene-Transfer Breakthrough
by D.L. Parsell | National Geographic News | January 11, 2002
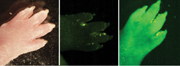
The paw of a mouse (in
ordinary light on the left) glows when seen under fluorescent light
(image on right), demonstrating that the animal's body cells contain a
gene from a jellyfish. The dark image in the center is of a paw of a
mouse that has not been "infected" with the jellyfish glowing material
and therefore it remains dark under fluorescent light. Researchers found
that the successful transfer of the jellyfish gene to mice made almost
all tissues of the animal fluorescent |
By using an innocuous virus derived from HIV,
scientists at the California Institute of Technology have developed a new way of
giving animals genes from other organisms to produce specific traits.
Termed transgenic animals, they are important to progress in biological research
and have a wide range of potential benefits in human health, agriculture, and
many other fields.
Animals that have been "engineered" to acquire certain medical conditions, for example, serve as good surrogates for studying human diseases and testing potential treatments and cures. In the future, cows might be given genes that enable them to produce milk containing therapeutic human proteins, or there may be transgenic chickens that can produce eggs low in cholesterol. Since the first transgenic animal, a mouse, was created more than two decades ago, different methods of development have been tried, but met with mixed success. The new method announced by the researchers at Caltech has some advantages over other techniques.
Today, transgenic animals are generally created by injecting "foreign" genes into the nucleus of animal cells—a procedure that is costly and requires a high degree of precision and expertise. The new technique entails using a powerful virus, much like the one that causes AIDS, to deliver the genes into the cells and insert them into an animal's genome.
"It's surprising how well it works," said David
Baltimore, a Nobel Prize-winning biologist who led the research team. "This
technique is much easier and more efficient than the procedure now commonly in
use, and the results suggest that it can be used to generate other transgenic
animal species."
Green Glow
The development of the new method, which was reported January 10 on the Web site Science Express, drew on Baltimore's pioneering research in the genetic makeup of viruses. Baltimore is now the president of Caltech.
In the Caltech experiments, the researchers stripped an HIV virus of its disease-causing elements and used it to virally infect single-cell embryos of mice with a gene from a jellyfish.
Any number of different genes could have been selected. For the purpose of the studies, the researchers chose a specific jellyfish gene that could serve as a "marker" to indicate whether the gene transfer was successful. The gene produces a protein that gives the jellyfish a green fluorescence.
When the mice were born, they carried the jellyfish gene in their own genes. Under fluorescent light, all their major tissues and organs—including skin, bones, muscles, lungs, liver, kidney, stomach, brain, and retina—emitted a green glow.
The trait became a permanent feature of the mice's genome and was passed along to many of their offspring.
Some of the mice glowed more than others, depending
on how many copies of the jellyfish gene they acquired. But 80 percent of the
original mice (14 out of 17) that received the gene transfer carried at least
one copy of the gene, and most of them, 90 percent, showed a high level of
fluorescence.
Trojan Horses, Gene Promoters
Carlos Lois, the lead author of a paper on the work, said the researchers used a modified HIV virus for the experiments to exploit the qualities that make HIV particularly virulent. Known as a retrovirus, HIV acts like a Trojan horse—once the virus infects cells, it transfers its own genetic material into the chromosomes of the host animal.
"How many horses do you let inside the cells? You can get one or many," said Lois, explaining why the mice could acquire one or multiple copies of the foreign gene from the invader virus. The scientists were able to control the level by manipulating the concentration of individual viruses present in the transfer solution.
A factor that apparently influences the results, Lois said, is the nature of the DNA material that controls how well a gene is "expressed," or manifested in certain traits.
The strings of chemicals that comprise individual genes are basically divided into two segments. One sequence carries the coding for making the proteins that produce various traits, while an accompanying sequence acts to "promote" the gene expression.
"If you have a very good promoter, one copy of the
virus may be sufficient" to produce an animal with the desired genetic trait,
Lois said, adding that the identification of reliable promoters is critical for
the successful application of transgenic technologies.
Toward Progress in Research
In the first experiments done to produce a transgenic animal, scientists used a different kind of virus to deliver the imported gene into mice. A major problem, however, was that the genes were "silenced" as the mice developed. "The approach was good and could have been used universally for many species, but there was no gene expression," said Lois.
In the 1980s, the research community adopted a technique called pronuclear injection—using tiny glass pipettes to insert genes from various organisms directly into DNA in the nucleus of mouse cells. Although it is now the most common method for producing transgenic animals, it is inefficient and limited mainly to mice, Lois noted, because 100 or more embryos may be required to achieve a single successful gene transfer.
"We have found that by using the modified virus we can combine the best of both these techniques because we can have expression of the gene and we are not limited by the number of embryos we have to obtain," Lois said.
The technique also holds much promise for inserting genes that regulate specific tissues of the body, Lois said. In one experiment, the researchers equipped the virus to carry, along with the jellyfish fluorescence gene, a gene and related promoter sequence that help regulate the development of muscles. When the researchers examined the mouse embryos at 11 days old, fluorescence was seen only in the nucleus of muscle cells and not other cells.
In another experiment, the mice were made to carry the jellyfish fluorescence gene along with a promoter sequence of DNA that regulates the development of lymphocytes, cells in blood and other tissue that aid the immune system. The resulting mice had the "green gene" expressed in blood cells.
"These results indicate that with this technique it is possible to direct the expression of exogenous genes into specific organs or tissues of the body," Lois said.
The researchers also generated some transgenic rats that successfully acquired the jellyfish fluorscence gene. This is a huge advantage to researchers, Baltimore noted, because rats are preferred for use in many laboratory experiments.
It should be possible, the scientists said, to use the new technique to produce many other transgenic animal species, especially higher mammals and birds.
"There's no real need for a new technique for mice, but there are many areas of research for which [transgenic] mice are not helpful—for example, as a model for Alzheimer's," said Lois. "So it would be extremely useful to develop monkeys that could develop Alzheimer's, or a primate that could get AIDS, so we could use them to test vaccines and treatments."
The Caltech team tried two different methods of inserting the virus and the fish gene it carried into the mice cells. One method involved injecting the virus under the layer (called the zona pellucida) that protects recently fertilized eggs. A second method entailed removing the protective layer and incubating the denuded fertilized eggs in a concentrated solution of the virus.The researchers found that the latter method was easier, but less efficient.
Like other methods of gene transfer, the viral-transmission technique has some limitations, the scientists noted. It may not work, for example, when transferring very large pieces of DNA.
